Anti-Hsp90 mAb [D7A]
产品名称: Anti-Hsp90 mAb [D7A]
英文名称: Hsp90 Monoclonal Antibody [D7A]
产品编号: SMC-137C
产品价格: null
产品产地: 加拿大
品牌商标: StressMarq
更新时间: 2023-09-21T00:39:30
使用范围: IP, WB, ELISA, IHC
- 联系人 :
- 地址 : 加拿大
- 邮编 :
- 所在区域 : 其他
- 电话 : 138****6620; 点击查看
- 传真 : 点击查看
- 邮箱 : hedyl@stressmarq.com
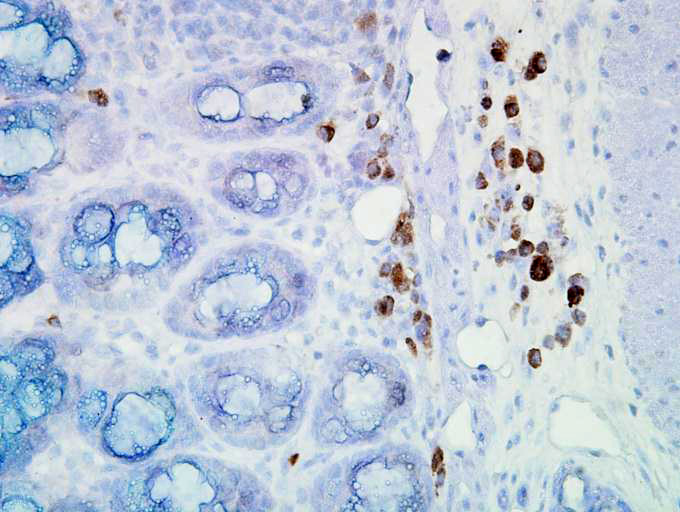
IHC staining of inflammatory cells in mouse colon tissue.
Hsp90 is a highly conserved and essential stress protein that is expressed in all eukaryotic cells. From a functional perspective, hsp90 participates in the folding, assembly, maturation, and stabilization of specific proteins as an integral component of a chaperone complex (4-7). Despite its label of being a heat-shock protein, hsp90 is one of the most highly expressed proteins in unstressed cells (1–2% of cytosolic protein). It carries out a number of housekeeping functions – including controlling the activity, turnover, and trafficking of a variety of proteins. Most of the hsp90- regulated proteins that have been discovered to date are involved in cell signaling (8-9). The number of proteins now known to interact with Hsp90 is about 100. Target proteins include the kinases v-Src, Wee1, and c-Raf, transcriptional regulators such as p53 and steroid receptors, and the polymerases of the hepatitis B virus and telomerase(6). When bound to ATP, Hsp90 interacts with co-chaperones Cdc37, p23, and an assortment of immunophilin-like proteins, forming a complex that stabilizes and protects target proteins from proteasomal degradation. In most cases, hsp90-interacting proteins have been shown to co-precipitate with hsp90 when carrying out immune-oadsorption studies, and to exist in cytosolic heterocomplexes with it. In a number of cases, variations in hsp90 expression or hsp90 mutation has been shown to degrade signaling function via the protein or to impair a specific function of the protein (such as steroid binding, kinase activity) in vivo. Ansamycin antibiotics, such as geldanamycin and radicicol, inhibit hsp90 function (10).
1. Schuh S. et al. (1985) J Biol Chem. 260 (26): 14292-14296.
2. Lipsich L.A., Cutt J.R. and Brugge J.S. (1982) Mol. Cell Biol. 2(7): 875-880.
3. Brugge J.S., Yonemoto W., and Darrow D. (1983) Mol. Cell. Biol. 3(1): 9-19.
4. Arlander SJH, et al. (2003) J Biol Chem 278: 52572-52577.
5. Pearl H, et al. (2001) Adv Protein Chem 59: 157-186.
6. Neckers L, et al. (2002) Trends Mol Med 8:S55-S61.
7. Pratt W, Toft D. (2003) Exp Biol Med 228:111-133.
8. Pratt W, Toft D. (1997) Endocr Rev 18: 306–360.
9. Pratt WB. (1998) Proc Soc Exptl Biol Med 217: 420–434.
10. Whitesell L, et al. (1994) Proc Natl Acad Sci USA 91: 8324– 8328.
近期发表文献
1. Akkad, H., Corpeno, R., Larsson L. (2014). Masseter muscle myofibrillar protein synthesis and degradation in an experimental critical illness myopathy model. PLoS One. 9(4): e92622. doi:10.1371/journal.pone.0092622
